
About Saypha Filler
Croma offers a complete range of Saypha Filler :
- saypha® RICH
- saypha® FILLER
- saypha® VOLUME
- saypha® VOLUME PLUS
The products are based on hyaluronic acid of non-animal origin and are manufactured in accordance with the highest quality standards.
Available with (CE 0123) and without (CE 0459) lidocaine
Range of Saypha Filler
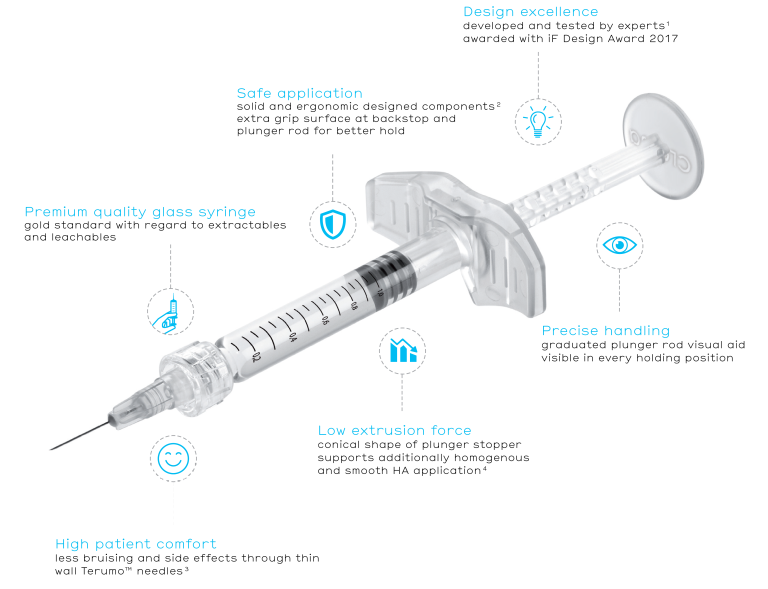
Croma Syringe
Innovation for safety and comfort

saypha® RICH
The device is a viscoelastic solution to replenish the loss of hyaluronic acid due to ageing, to maintain hydration, improve tone and elasticity of the skin and to act as a filler for small lines such as crow‘s feet, smile lines or smoke lines surrounding the mouth.

saypha® FILLER
For correcting moderate to severe nasolabial folds. Used for cosmetic and medical reconstructive purposes in the treatment of e.g. facial lipoatrophy, debilitating scars or morphological asymmetry

saypha® VOLUME
For correcting moderate to severe nasolabial folds and medical reconstructive treatment, for instance, of facial lipoatrophy, debilitating scars, or morphological asymmetry

saypha® VOLUME PLUS
The intended purpose of the device is to restore facial volume in order to correct moderate to severe midface volume deficit to treat signs of ageing.
About Saypha Filler
Crosslinked hyaluronic acid with 0.3% lidocaine
Saypha FILLER Lidocaine
Crosslinked hyaluronic acid with 0.3% lidocaine
Intended purpose : To create volume in order to correct wrinkles and folds in order to treat signs of ageing Indication : The device is indicated to correct moderate to severe nasolabial folds and to increase lip volume. Injection area : Phosphate buffer, NaCl, 0.3% lidocaine hydrochloride
Concentration HA : 2.3 % (23mg/mL) high molecular weight hyaluronic acid of non-animal origin Crosslinking agent : BDDE (concentration ≤ 2 ppm) Additional ingredients : Phosphate buffer, NaCl, 0.3% lidocaine hydrochloride
Packaging unit : 1 box of 1mL syringe Needle : 2×27G ½” thin wall TerumoTM needles (CE 0197)
Est. duration in the skin : Anticipated at least 9 months
Saypha VOLUME Lidocaine
Crosslinked hyaluronic acid with 0.3% lidocaine
Intended purpose : To create volume in order to correct wrinkles and folds in order to treat signs of ageing. Indication : The device is indicated to correct moderate to severe nasolabial folds. Injection area : Deep dermis or subcutis
Concentration HA : 2.3% (23mg/mL) high molecular weight hyaluronic acid of non-animal origin
Crosslinking agent : BDDE (concentration ≤ 2 ppm)
Additional ingredients : Phosphate buffer, NaCl, 0.3% lidocaine hydrochloride
Packaging unit : 1 box of 1mL syringe Needle : 2×27G ½” thin wall TerumoTM needles (CE 0197)
Est. duration in the skin : Anticipated at least 9 months
Saypha VOLUME PLUS Lidocaine
Crosslinked hyaluronic acid with 0.3% lidocaine
Intended purpose : The intended purpose of the device is to restore facial volume in order to correct moderate to severe midface volume deficit to treat signs of ageing. Indication : The device is indicated to correct moderate to severe midface volume defi cit in the zygomaticomalar region, anteromedial cheek region and submalar region. Injection area : Deep subcutaneous and/or supraperiosteal
Concentration HA : 2.5% (25mg/mL) high molecular weight hyaluronic acid of non-animal origin Crosslinking agent : BDDE (concentration ≤ 2 ppm) Additional ingredients : Phosphate buffer, NaCl, 0.3% lidocaine hydrochloride
Packaging unit : 1 box of 1mL syringe Needle : 2×27G ½” thin wall TerumoTM needles (CE 0197)
Est. duration in the skin : Anticipated at least 9 months
